
精微高博公众号
北京精微高博仪器有限公司
Sales@jwgb.net
 比表面积分析仪
比表面积分析仪
 介孔孔径分析仪
介孔孔径分析仪 微孔孔径分析仪
微孔孔径分析仪 化学吸附仪
化学吸附仪 反应评价装置
反应评价装置 蒸汽吸附仪
蒸汽吸附仪 穿透曲线分析仪
穿透曲线分析仪 真密度仪
真密度仪 压汞仪
压汞仪 高压吸附仪
高压吸附仪 热分析仪
热分析仪 X射线衍射仪
X射线衍射仪 脱气机
脱气机 质谱仪
质谱仪 相关配件
相关配件 比表面积分析仪
比表面积分析仪
 介孔孔径分析仪
介孔孔径分析仪
 微孔孔径分析仪
微孔孔径分析仪
 化学吸附仪
化学吸附仪
 反应评价装置
反应评价装置
 蒸汽吸附仪
蒸汽吸附仪
 穿透曲线分析仪
穿透曲线分析仪
 真密度仪
真密度仪
 压汞仪
压汞仪
 高压吸附仪
高压吸附仪
 热分析仪
热分析仪
 X射线衍射仪
X射线衍射仪
 脱气机
脱气机
 质谱仪
质谱仪
 相关配件
相关配件

paper
Gang-Tian Zhu,† Xiao-Mei He,‡ Sheng He,† Xi Chen,§ Shu-Kui Zhu,∥ and Yu-Qi Feng*,‡
†Key Laboratory of Tectonics and Petroleum Resources (Ministry of Education), China University of Geosciences, Wuhan 430074, P.R. China
‡Key Laboratory of Analytical Chemistry for Biology and Medicine (Ministry of Education), Department of Chemistry, Wuhan
University, Wuhan 430072, P.R. China
§Wuhan Institute of Biotechnology, Wuhan 430072, P.R. China
∥State Key Laboratory of Biogeology and Environmental Geology, China University of Geosciences, Wuhan 430074, P.R. China
1. INTRODUCTION
Mesoporous silica has found a great utility in sample preparation because of its high surface area, accessible mesopores and modifiable surface properties.1,2 As an adsorbent, mesoporous structure and functionalized group are regarded as two essential factors for the application.3
Mesoporous structure contributes large surface area for the adsorbent. Functionalizations enable silica materials meet the demands of selective adsorption of special compounds.
Organic silica hybrid mesoporous materials represent one important class of functionalized mesoporous silica materials.4
These hybrid materials can be prepared by subsequent attachment of organofunctional groups onto pure silica matrices (postgrafting method), or by cross-linking cocondensation reaction of inorganic silicate and organic functional species (co-condensation method).5 It should be noted that the distribution of organic functional units in hybrid materials prepared by co-condensation method are generally more homogeneous than that prepared by postgrafting method.6
Apart from mesoporous structure and functionalized group, particle size is also crucial to the mesoporous adsorbent, which can determine the operation format in sample preparation.7
Nanoparticles are good adsorbents in dispersive solid phase extraction (SPE), in which adsorbents are collected from sample solution by centrifugation.8,9 Materials with large particle size can serve as adsorbents in packed SPE apparatuses such as in-cartridge,10 in-syringe,11 or in-pipet-tip format12 with low back-pressure. For instance, the particle sizes of the adsorbents in commercial Zip-Tip (Millipore) and Supel-Tip (Supelco) are about 15 and 50 μm, respectively. Notably, unlike dispersive SPE, these packed SPE assays are rapid and easy to operate without time-consuming centrifugation steps.
To date, preparation of functionalized mesoporous silica (FMS) with particle size ranged from nanometer to submicrometer has been reported in many previous works.13
However, synthesis of FMS with particle size ranged from tens micrometer to submillimeter remains a challenge.14 It is difficult to achieve large particle size, mesoporous structure and homogeneous functionalization simultaneously.
Phosphoproteins play vital roles in numerous physiological and pathological processes.15,16 Because of the complexity of biological sample, specific enrichment of the low abundance of phosphopeptides in the enzymatic digests of phosphoproteins as well as endogenous phosphopeptides in biological samples is indispensable prior to mass spectrometry analysis.17,18 Because of the negative charge of phosphopeptides, anion-exchange chromatography (AEX) has been one method to enrich phosphopeptides.19 But development of AEX material to highly specific enrichment of phosphopeptides is still less studied as compared to other methods such as immobilized metal affinity chromatography (IMAC) or metal oxide affinity chromatography (MOAC). On the other hand, because of the extreme complexity of proteome sample, single affinity material cannot achieve complete recovery and different affinity materials have different biases on subsets of phosphopeptides.20 It means that development of new affinity materials may yield complementary or superior results to the existing approaches.
Here, we designed a facile method for synthesis of polyethylenimine (PEI) functionalized mesoporous silica (PFMS) and utilized it as an AEX material for phosphopeptide enrichment. PFMS was synthesized by a co-condensation method, in which PEI served as four roles simultaneously including functionalized reagent, alkaline catalyst, template for particle formation, and pore-structure-directing agent. The prepared PFMS possessed large particle size and high surface area, making it a capable candidate for convenient packed SPE.
Additionally, profited from the abundant amine groups in the organic units, PFMS was packed in a pipet tip for rapid capture of phosphopeptides. The performance of the in-pipet-tip method was investigated by enrichment of phosphopeptides from tryptic digests of standard protein mixtures, tryptic digests of nonfat milk, human serum and tryptic digests of rat brain lysate.
2. EXPERIMENTAL SECTION
2.1. Chemicals and Materials. Tetraethyl orthosilicate (TEOS), ethanol (EtOH), cetyltrimethylammonium bromide (CTAB), orthophosphoric acid (H3PO4, 85 wt % in H2O), formic acid (FA), ammonium nitrate (NH4NO3), and ammonia solution (NH3·H2O, 25 wt % in H2O) were purchased from Shanghai General Chemical Reagent Factory (Shanghai, China). Cotton wool was purchased from Xuzhou Hygiene of Material Factory (Xuzhou, China). HPLC grade acetonitrile (ACN) was supplied by Fisher Scientific (Pittsburgh, USA). Commercial TiO2 (T104936) was supplied by Aladdin Chemical Reagent (Shanghai, China). Polyethylenimine (PEI, MW 70 kDa, 50 wt % in H2O) was purchased from Aladdin Chemical Reagent (Shanghai, China). Bovine β-casein, bovine serum albumin (BSA), trifluoroacetic acid (TFA), and 2,5-dihydroxybenzoic acid (2,5- DHB) were supplied by Sigma-Aldrich (St Louis, USA). Sequencing grade trypsin was supplied by Promega (Madison, USA). Purified
water was produced by a Milli-Q apparatus (Millipore, Bedford, USA).
Nonfat milk was supplied by a local supermarket. Zip-Tip C18 was purchased from Merck Millipore (Darmstadt, Germany). Healthy human serum was gained from Wuhan Zhongnan Hospital by standard clinical procedures.
2.2. Preparation of Polyethylenimine Functionalized Mesoporous Silica (PFMS). First, CTAB (0.5 g) was dissolved in 15 mL of EtOH/H2O (1/1, v/v). After TEOS (15 g) was added to the mixture, 200 μL of H3PO4 was added dropwise to the solution. The mixture was stirred at room temperature for 2 h to form a transparent silica sol.
Then PEI aqueous solution was added to the sol with continuous stirring. The effect of mass ratio of PEI/TEOS in a range of 0.04−0.3 (x) on the property of the product (PFMS-x) was investigated. After reaction for 3 h, the product was washed repeated with H2O and
EtOH. Finally, the particles were redispersed in 50 mL of NH4NO3 ethanol solution (0.2 wt %) and heated at 60 °C for 0.5 h to remove
CTAB. The step was repeated six times, and the purified product was washed with H2O for 2 times to obtain PFMS-x. As a comparison, pure mesoporous silica without PEI was prepared by using 3 mL of ammonia solution as the alkaline catalyst instead of PEI, while the other details were the same as the synthesis procedure of PFMS.
2.3. Characterization. Scanning electron microscopy (SEM) images were obtained by a Quanta-200 scanning electron microscope (FEI, Holland). Thermogravimetric analysis (TGA) was carried out on a NETZSCH STA409PC thermal analyzer (Bavaria, Germany) under air flowing. Elemental analysis was performed on a Vario EL III elemental analyzer (Elementar, Germany). Nitrogen sorption experiments were measured at 77 K with a JW-BK surface area and porosity analyzer (JWGB Sci. & Tech., China). The materials were pretreated
by vacuum heating at 100 °C for 6 h to eliminate the physically adsorbed substances prior to analysis. The value of specific surface area was calculated from the sorption isotherm with P/P0 from 0.05 to 0.3 according to Brunauer−Emmett−Teller equation. The pore structure parameters were evaluated from the desorption isotherm by using
Barrett−Joyner−Halenda model.
2.4. Phosphopeptide Enrichment. PFMS (1 mg) was packed in a 200 μL-pipet-tip between two layers of otton, and the in-pipet-tip system was directly used for phosphopeptide enrichment. Briefly, sample solution (50 μL) was aspirated and dispensed 20 cycles to allow phosphopeptides to be adsorbed onto the PFMS. After washing twice by loading buffer (50 μL), the adsorbed peptides were eluted by ACN/5% NH3·H2O (1/1, v/v, 50 μL). The eluate solution was lyophilized to dryness and redissolved in matrix solution (2 μL) (20 mg/mL of 2, 5-DHB in ACN/2% H3PO4, 1/1, v/v), and 1 μL of the solution was applied to MALDI-TOF-MS analysis.
For phosphopeptide enrichment from tryptic digests of nonfat milk, 1000-fold dilution of the digests in loading buffer (ACN/0.2%FA, 1/1, v/v) was used for extraction. For endogenous phosphopeptide enrichment from human serum, 10-fold dilution of human serum in loading buffer was used for extraction.
For phosphopeptide enrichment from tryptic digests of rat brain lysate, 0.5 mg of the digests was redissolved in 50 μL of loading buffer (ACN/0.2% FA, 1/1, v/v) as sampling solution. The eluate was lyophilized to dryness and redissolved in 10 of H2O. The solution was desalted by Zip-Tip C18 and applied to RPLC-ESI-MS/MS analysis.
For comparison, commercial TiO2 was also used to enrich phosphopeptides from tryptic digests of rat brain lysate. The detailed procedures were shown in Supporting Information.
2.5. Mass Spectrometry Analysis. MALDI-TOF-MS analysis was performed on an Axima TOF2 mass spectrometer (Shimadzu, Kyoto, Japan). RPLC-ESI-MS/MS analysis was performed on a TripleTOF 5600+ mass spectrometer (AB Sciex, Massachusetts, USA). The detailed conditions of the two MS analyses were shown in Supporting Information.
3. RESULTS AND DISCUSSION
3.1. Preparation and Characterization of PFMS. The preparation procedure is shown in Scheme 1. Briefly, TEOS Scheme 1. Formation of PFMS
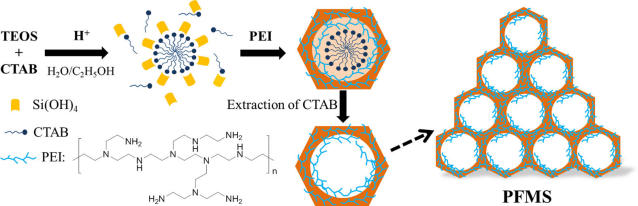
was hydrolyzed and cooperatively assembled with the surfactant CTAB.21 Then PEI was added to initiate and accelerate the polymerization and condensation of inorganics and organics.
Finally, CTAB templates were removed in a mild way, andPFMS was obtained.
The effect of the mass ratio of PEI/TEOS from 0.04 to 0.3 (x) on the property of the product (PFMS-x) was investigated (Table 1). As a comparison, pure mesoporous silica without
Table 1. Surface Areas, Pore Diameters, Weight Losses in
TGA, and N Content of Different Products
sample |
surface area (m2 g−1) | pore diameter (nm) |
weight loss in TGA (wt %) |
N content (wt %) |
PFMS-0.04 | 487 | 5.4 | 9.7 | 2.5 |
PFMS-0.1 | 404 | 5.4 | 15.8 | 4.3 |
PFMS-0.2 | 286 | 5.3 | 24.4 | 7.2 |
PFMS-0.3 | 261 | 5.3 | 30.4 | 9.3 |
mesoporous | 632 | 3.3 | 2.7 | a |
silica
aNot detected.
PEI was prepared by using ammonia solution as the alkaline catalyst. According to the SEM images, the shapes and izes of
PFMS-x (Figure 1a−d) are distinctly different from those of pure mesoporous silica (Figure 1e and f). When the mass ratio of PEI/TEOS is between 0.1 and 0.3, the particle sizes of the products range from 20 to 100 μm, which are eligible to serve as adsorbents of packed SPE; when the mass ratio of PEI/TEOS is 0.04, some of the particle sizes are around 5 μm, while most of the particle sizes are above 20 μm. Whereas, when ammonia solution was used as the alkaline catalyst instead of PEI, the products were spheres with an average size of about 0.6 μm (Figure 1f). These results indicate that PEI played a significant role in the formation of product with large particle size.
As for the formation mechanism (Scheme 1), small amounts of moderate strong acid (H3PO4) were first used to prehydrolyze TEOS into inorganic silicates. Both the literature13 and our experimental phenomenon revealed that the prehydrolyzed-silicate sols catalyzed by H3PO4 were stable and no precipitation would be produced, probably because the pH of this H3PO4-containing system was around the isoelectric point of silica (pH ≈ 2.0), in which the condensation of silicates was extremely slow.22 The stable silicate-surfactant nucleis were ready for the fast condensation of silica after addition of alkaline catalyst. Then, PEI was added as the alkaline catalyst and template for particle formation. PEI is a cationic polymer that has been used as one of the most notable gene vectors.23,24 As shown in Scheme 1, PEI contains a protonatable amino nitrogen at every third atom of the polymeric backbone, make it highly basic and positive charged.25,26 The pH of the PEI aqueous solution used in this work was about 10. Consequently, PEI can act as the alkaline catalyst in condensation of silica. Besides, the high reactive primary and secondary amino group in the polymer chain of PEI make it a good cross-linking agent; and because of the high molecular weight (70 kDa) and gelation tendency in the presence of acid,27 PEI is a favorable template to generate large particle when co-condensing with silica.

Figure 1. SEM images of PFMS-0.04 (a), PFMS-0.1 (b), PFMS-0.2 (c), PFMS-0.3 (d), and pure mesoporous silica (e, f).
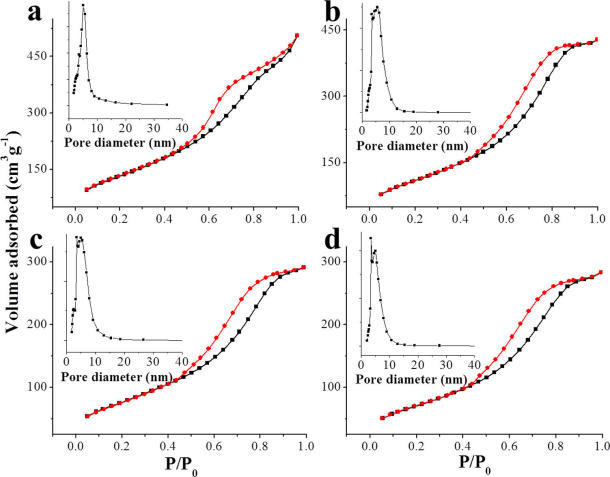
Figure 2. N2 sorption−desorption isotherms and mesopore size distributions (the insets) of PFMS-0.04 (a), PFMS-0.1 (b), PFMS-0.2 (c), and
PFMS-0.3 (d).
The organic contents of the products were determined by thermogravimetric analysis (TGA). The weight loss of PFMS-x ranges from 9.7 to 30.4 wt % and increases with the mass ratio of PEI/TEOS, while a weight loss of only 2.7 wt % is obtained for pure mesoporous silica catalyzed by ammonia solution (Table 1). The N contents of the products were measured by elemental analysis. For PFMS-x, the N content ranges from 2.5 to 9.3 wt % and increases with the mass ratio of PEI/TEOS (Table 1). On the contrary, there was no assignable N content detected in pure mesoporous silica. These results indicate the successful functionalization of PEI in PFMS. Notably, since PEI was functionalized by co-condensation with silica, the organic units in PFMS would be more homogeneously distributed than that in other FMS synthesized by postgrafting method, especially when the particle size was large.4
The surface area and pore parameters of the materials were assessed by N2 sorption measurement. The N2 sorption desorption isotherms show IV-type curves for all the products (Figure 2; Figure S1), indicating the presence of mesostructure.
The surface area of PFMS-x decreases with the increased mass ratio of PEI/TEOS, and all the surface areas of PFMS-x are lower than that of pure mesoporous silica (Table 1). Moreover, the pore size distributions of PFMS-x are different from that of pure mesoporous silica. Typically, the pore size distributions of PFMS-x are similar, with a mean value of 5.4 or 5.3 nm (Figure 2, inset; Table 1), while the pore size distribution of puremesoporous silica exhibits a sharp peak with a mean value of 3.3 nm (Figure S1, inset). These results show that PEI participated in the formation of mesostructure. Indeed, because of the hydrophobic vinyl group and positive charged amino group,PEI is a cationic surfactant and can involve in the formation of mesostructure together with CTAB.
3.2. Phosphopeptide Enrichment with PFMS Using In-Pipet-Tip SPE. Considering the particle size, surface area, andPEI content, PFMS-0.3 was chosen as the representative material for further application. The large particle size enables PFMS to serve as an adsorbent for packed SPE with low backpressure; the high surface area allows the employ of small amounts of adsorbents with sufficient extraction capacity in a miniaturized SPE;28 the extremely abundant basic sites in the organic units of PFMS make it a promising anion-exchanger for sample preparation.
Here, we attempt to explore the practicability for phosphopeptide enrichment by using PFMS as an anionexchanger, in view of the abundant basic sites of PFMS. PFMS was packed in a 200-μL-pipet tip with cotton on both ends and used for in-pipet-tip enrichment of phosphopeptides (Figure 3). The aspiration and dispensation of solution with the inpipettip system was fast and smooth with low back-pressure,benefiting from the large particle size of the adsorbent.
To study the extraction mechanism and optimize the extraction condition, PFMS was first utilized to enrich phosphopeptides from tryptic digests of β-casein in six different loading buffers. Figure S2a shows that when the loading buffer was pure water, phosphopeptides as well as nonphosphopeptides were trapped onto PFMS. When the loading buffer was changed to ACN/H2O (1/1, v/v) or ACN/0.02% FA (1/1, v/v), the specificity was improved, but the signals of nonphosphopeptides were still obvious (Figure S2b, c). When the loading buffer was ACN/0.1% FA (1/1, v/v) or ACN/0.2% FA(1/1, v/v), 3 or 4 phosphopeptides were detected (Figure S2d,e and Table S1), respectively. Further increasing the acid content resulted in loss of phosphopeptide signals (Figure S2f).These results reveal that there exists remarkable ion-exchange interaction between PFMS and phosphopeptides; and ACN/0.2% FA (1/1, v/v) was selected as the loading buffer for further phosphopeptide enrichment. Under the optimized conditions, low concentrations of tryptic digests of β-casein (50 μL) were used to examine the sensitivity of the method. As shown in Figure S3, even when the concentration is 2 × 10−10 M (the amount is 10 fmol), one phosphopeptide can still be detected with signal-to-noise ratio above 3, indicating the high sensitivity of the method, which is comparable with our previous works.18,29

Figure 3. Photographic image of the PFMS packed pipet tip for phosphopeptide enrichment.
The specificity of PFMS was investigated by capture of phosphopeptides from three complex samples, including tryptic digests of standard protein mixtures, tryptic digests of nonfat milk, and human serum. Figure 4a, c, and e show the spectra for direct analyses of the mixtures of tryptic digests of β-casein and BSA with molar ratios of 1:1, 1:10, and 1:100. With the increase of the ratio of BSA digestion, nonphosphopeptide signals dominated the spectra, and detection of phosphopeptides became impossible. After enrichment by PFMS, 4 phosphopeptides were clearly detected with excellent resolution (Figure 4b,d, and f, Table S1). As for the direct analysis of tryptic digests of nonfat milk, only 7 phosphopeptides were detected, which were suppressed by many dominant signals of nonphosphopeptides (Figure 5a). Whereas, after enrichment by PFMS, 19 phosphopeptides were identified with very few interferences (Figure 5b and Table S1). Furthermore, due to the complex matrix of human serum, direct analysis generated no peptide signals (Figure 5c). This scenario could be significantly changed by the treatment with PFMS. As a result, 4 phosphopeptides were clearly isolated and detected (Figure 5d and Table S2). These results indicate that PFMS possesses prominent specificity toward phosphopeptides in complex samples.
Encouraged by the outstanding performance of PFMS, the in-pipet-tip approach was further applied to enrich phosphopeptides from a real complex rat tissue sample (tryptic digests of rat brain lysate) followed by RPLC-ESI-MS/MS analysis. Commercial TiO2 was used for the comparison. Both of the extraction experiments with the two materials were repeated three times. To the extraction experiments with PFMS, 2018, 2071, and 2089 unique phosphopeptides were detected in the triplicate tests, respectively. The overlap of the three results is shown in Figure 6a. A total of 2251 unique phosphopeptides were detected (Table S3), in which 1913 phosphopeptides (85.0% of the total pool of Figure 6a) were detected in all the triplicate tests. To the extraction experiments with TiO2, 1291,1345, and 1388 unique phosphopeptides were detected in the triplicate tests, respectively. The overlap of the three results is shown in Figure 6b. A total of 1520 unique phosphopeptides were detected (Table S4), in which 1211 phosphopeptides (79.7% of the total pool of Figure 6b) were detected in all the triplicate tests. The high degree of overlap among the triplicate tests with PFMS, which is slightly higher than that with TiO2, predicts the good reproducibility of the method. In addition, the ratio of the number of phosphopeptides to that of whole peptides is described as the assessment value of specificity. The average values of specificity with PFMS and TiO2 are 77.6% and 70.2%, respectively, which reveal that the specificity of PFMS is a bit higher than that of TiO2. More importantly, only 268 phosphopeptides (7.7% of the total pool of Figure 6c) were identified by both of the two adsorbents, demonstrating that the two methods are complementary. These results confirm that the in-pipet-tip approach with PFMS as adsorbent owns great application value in phosphopeptide enrichment from complex biological samples.
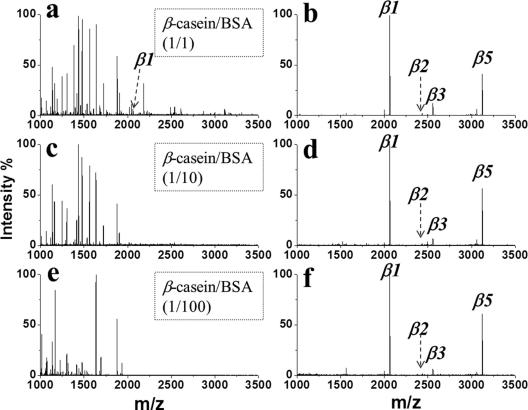
Figure 4. MALDI mass spectra obtained by direct analysis (a, c, e) or after enrichment by PFMS (b, d, f) from the tryptic digest of a mixture of β-casein/BSA with the ratio of 1:1 (a, b), 1:10 (c, d), or 1:100 (e, f). The concentration of tryptic digests of β-casein was 1.0 × 10−7 M. The phosphopeptides are marked with “βn”.
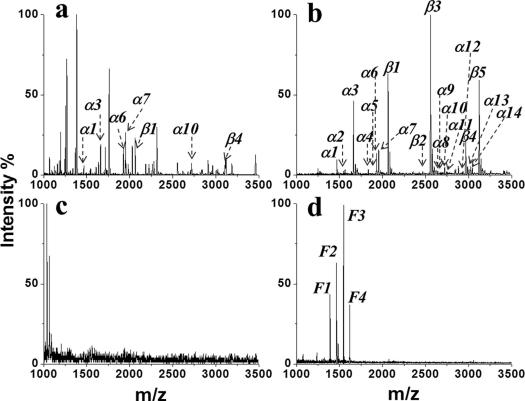
Figure 5. MALDI mass spectra of tryptic digests of nonfat milk prior to enrichment (a) and after enrichment by PFMS (b). MALDI mass spectra of human serum before enrichment (c) and after enrichment by PFMS (d). The phosphopeptides are marked with “βn”, “αn”, or “Fn”.
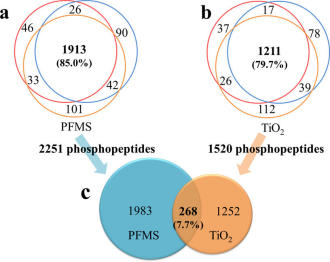
Figure 6. Venn diagrams showing the overlap of identified phosphopeptides from tryptic digests of rat brain lysate among the triplicate tests with PFMS (a) or TiO2 (b) and the overlap of the whole identified phosphopeptides with PFMS and TiO2(c).
4. CONCLUSIONS
In conclusion, we have developed a facile method for synthesis of polyethylenimine functionalized mesoporous silica (PFMS) with large particle size and high surface area. The abundant basic sites make PFMS a promising anion-exchanger. It was found that PFMS can be used to rapid, convenient and specific enrich phosphopeptides from complex biological samples withan in-pipet-tip approach. We believe that PFMS has great potential as an adsorbent or catalyst support, and this work may provide inspirations for development of other functionalized mesoporous silica with large particle size.
■ ASSOCIATED CONTENT
*S Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsami.6b10948.
Preparation of proteome samples; N2 sorption−desorption isotherms and mesopore size distribtuion of pure mesoporous silica; MALDI mass spectra of tryptic digests of β-casein after enrichment with PFMS in different loading buffers; MALDI mass spectrum of tryptic digests of β-casein with concentration of 2.0 × 10−10 M after enrichment with PFMS; detailed information on the identified phosphopeptides from tryptic digests of β-casein, tryptic digests of nonfat milk, human serum, and tryptic digests of rat brain lysate (PDF)
■ ACKNOWLEDGMENTS
The authors are grateful for financial support from the National Basic Research Program of China (973 Program) (2013CB910702) and the Fundamental Research Funds for the Central Universities, China University of Geosciences (Wuhan) (CUG160606).
■ REFERENCES
(1) Sierra, I.; Perez-Quintanilla, D. Heavy Metal Complexation on Hybrid Mesoporous Silicas: An Approach to Analytical Applications.Chem. Soc. Rev. 2013, 42 (9), 3792−3807.
(2) Zhao, L.; Qin, H.; Wu, R.; Zou, H. Recent Advances of Mesoporous Materials in Sample Preparation. J. Chromatogr. A 2012, 1228, 193−204.
(3) Wu, Z.; Zhao, D. Ordered Mesoporous Materials as Adsorbents. Chem. Commun. 2011, 47 (12), 3332−3338.
(4) Hoffmann, F.; Cornelius, M.; Morell, J.; Froba, M. Silica-Based Mesoporous Organic-Inorganic Hybrid Materials. Angew. Chem., Int. Ed. 2006, 45 (20), 3216−3251.
(5) Stein, A.; Melde, B. J.; Schroden, R. C. Hybrid Inorganic-Organic Mesoporous Silicates - Nanoscopic Reactors Coming of Age. Adv. Mater. 2000, 12 (19), 1403−1419.
(6) Lim, M. H.; Stein, A. Comparative Studies of Grafting and Direct Syntheses of Inorganic-Organic Hybrid Mesoporous Materials. Chem. Mater. 1999, 11 (11), 3285−3295.
(7) Liu, Q.; Shi, J.; Sun, J.; Wang, T.; Zeng, L.; Jiang, G. Graphene and Graphene Oxide Sheets Supported on Silica as Versatile and High-Performance Adsorbents for Solid-Phase Extraction. Angew. Chem., Int.Ed. 2011, 50 (26), 5913−5917.
(8) Tian, R.; Zhang, H.; Ye, M.; Jiang, X.; Hu, L.; Li, X.; Bao, X.; Zou, H. Selective Extraction of Peptides from Human Plasma by Highly Ordered Mesoporous Silica Particles for Peptidome Analysis. Angew.Chem., Int. Ed. 2007, 46 (6), 962−965.
(9) Qi, Y. X.; Wu, D. P.; Wei, J. Y.; Ding, K.; Wang, H.; Zhang, Y. J.;Qian, X. H.; Guan, Y. F. Selective Extraction of Low Molecular Weight Proteins by Mesoporous Silica Particles with Modified Internal and External Surfaces. Anal. Bioanal. Chem. 2010, 398 (4), 1715−1722.
(10) Luo, Y. B.; Zhu, G. T.; Li, X. S.; Yuan, B. F.; Feng, Y. Q. Facile Fabrication of Reduced Graphene Oxide-Encapsulated Silica: A Sorbent for Solid-Phase Extraction. J. Chromatogr. A 2013, 1299,10−17.
(11) Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Miniaturized Solid-Phase Extraction Techniques. TrAC, Trends Anal. Chem. 2015, 73, 19−38.
(12) Atakay, M.; Çelikbıçak, Ö.; Salih, B. Amine-Functionalized Sol Gel-Based Lab-in-a-Pipet-Tip Approach for the Fast Enrichment and Specific Purification of Phosphopeptides in MALDI-MS Applications.Anal. Chem. 2012, 84 (6), 2713−2720.
(13) Wu, S. H.; Mou, C. Y.; Lin, H. P. Synthesis of Mesoporous Silica Nanoparticles. Chem. Soc. Rev. 2013, 42 (9), 3862−3875.
(14) Galarneau, A.; Iapichella, J.; Bonhomme, K.; Di Renzo, F.;Kooyman, P.; Terasaki, O.; Fajula, F. Controlling the Morphology of
Mesostructured Silicas by Pseudomorphic Transformation: A Route Towards Applications. Adv. Funct. Mater. 2006, 16 (13), 1657−1667.
(15) Riley, N. M.; Coon, J. J. Phosphoproteomics in the Age of Rapid and Deep Proteome Profiling. Anal. Chem. 2016, 88 (1), 74−94.
(16) Zou, Y.; Wang, Y. S. Mass Spectrometric Analysis of High-Mobility Group Proteins and Their Post-Translational Modifications
in Normal and Cancerous Human Breast Tissues. J. Proteome Res.2007, 6 (6), 2304−2314.
(17) Zhao, L.; Qin, H.; Hu, Z.; Zhang, Y.; Wu, R. a.; Zou, H. A Poly(Ethylene Glycol)-Brush Decorated Magnetic Polymer for Highly Specific Enrichment of Phosphopeptides. Chem. Sci. 2012, 3 (9),2828−2838.
(18) He, X. M.; Chen, X.; Zhu, G. T.; Wang, Q.; Yuan, B. F.; Feng, Y.Q. Hydrophilic Carboxyl Cotton Chelator for Titanium(IV) Immobilization and Its Application as Novel Fibrous Sorbent for Rapid Enrichment of Phosphopeptides. ACS Appl. Mater. Interfaces 2015, 7 (31), 17356−17362.
(19) Zhu, G. T.; He, X. M.; Chen, X.; Hussain, D.; Ding, J.; Feng, Y.Q. Magnetic Graphitic Carbon Nitride Anion Exchanger for Specific Enrichment of Phosphopeptides. J. Chromatogr. A 2016, 1437, 137144.
(20) Wang, F.; Song, C.; Cheng, K.; Jiang, X.; Ye, M.; Zou, H.Perspectives of Comprehensive Phosphoproteome Analysis Using Shotgun Strategy. Anal. Chem. 2011, 83 (21), 8078−8085.
(21) Li, W.; Zhao, D. An Overview of the Synthesis of Ordered Mesoporous Materials. Chem. Commun. 2013, 49 (10), 943−946.
(22) Wan, Y.; Zhao. On the Controllable Soft-Templating Approach to Mesoporous Silicates. Chem. Rev. 2007, 107 (7), 2821−2860.
(23) Peng, Q.; Chen, F.; Zhong, Z.; Zhuo, R. Enhanced Gene Transfection Capability of Polyethylenimine by Incorporating Boronic Acid Groups. Chem. Commun. 2010, 46 (32), 5888−5890.
(24) Vicennati, P.; Giuliano, A.; Ortaggi, G.; Masotti, A.Polyethylenimine in Medicinal Chemistry. Curr. Med. Chem. 2008,15 (27), 2826−2839.
(25) Tripathi, S. K.; Ahmadi, Z.; Gupta, K. C.; Kumar, P.Polyethylenimine-Polyacrylic Acid Nanocomposites: Type of Bonding Does Influence the Gene Transfer Efficacy and Cytotoxicity. Colloids Surf., B 2016, 140, 117−120.
(26) Cai, H.; An, X.; Cui, J.; Li, J.; Wen, S.; Li, K.; Shen, M.; Zheng, L.; Zhang, G.; Shi, X. Facile Hydrothermal Synthesis and Surface Functionalization of Polyethyleneimine-Coated Iron Oxide Nanoparticles for Biomedical Applications. ACS Appl. Mater. Interfaces 2013,5 (5), 1722−1731.
(27) Noh, M.; Kang, S.; Mok, Y.; Choi, S. J.; Park, J.; Kingma, J.; Seo,J. H.; Lee, Y. Upper Critical Solution Temperature (Ucst) Phase Transition of Halide Salts of Branched Polyethylenimine and Methylated Branched Polyethylenimine in Aqueous Solutions. Chem.Commun. 2016, 52 (3), 509−512.
(28) Chigome, S.; Darko, G.; Torto, N. Electrospun Nanofibers as Sorbent Material for Solid Phase Extraction. Analyst 2011, 136 (14),2879−2889.
(29) He, X. M.; Zhu, G. T.; Zhu, Y. Y.; Chen, X.; Zhang, Z.; Wang, S.T.; Yuan, B. F.; Feng, Y. Q. Facile Preparation of Biocompatible Sulfhydryl Cotton Fiber-Based Sorbents by ″Thiol-Ene″ Click Chemistry for Biological Analysis. ACS Appl. Mater. Interfaces 2014,6 (20), 17857−17864.